It refers to applications of Biology in combination with Chemistry to produce products, methods or services that would benefit humanity.
1.) Genetic Engineering- manipulation of genes of organisms using recombinant DNA.
2.) Recombinant DNA- refers to the insertion of DNA segments into another DNA to produce hybrid gene.
3.) The Human Genome Project- will identify the DNA bases located in the entire genetic material in human beings.
4.) Transgenic Animals- are used to produce medically important proteins with commercial values.
5.) Stem Cells Technology- could treat human diseases.
6.) Bioremediation- use biotechnology to solve environmental problems, use of natural and recombinant microorganisms to breakdown toxic and hazardous substance already present in the environment.
7.) Gene Therapy- indetification and repair of mutated genes.
Note: Antigen is a harmful organism.
http://www.odofin.com/english/short.htm "TRIP" (I'm just trying to make your nose bleed) LOL! :DD
Let's move on to...
VI. Organic Compounds
Carbohydrates
- chief energy source of organisms
Simple Sugars or "Monosaccharides": Glucose, Fructose and Galactose
Double Sugars or "Disaccharides":
Glucose + Glucose= Maltose
Glucose + Fructose= Sucrose
Glucose + Galactose= Lactose
Polysaccharides
Starch- main stored food in plants
Glycogen- main stored food in animals
Cellulose- forms part of the wall that encloses plant cell.
Isomer- compounds that have the same formula.
Lipids
*The lipids are a large and diverse group of naturally occurring organic compounds that are related by their solubility in nonpolar organic solvents (e.g. ether, chloroform, acetone & benzene) and general insolubility in water.
*large molecules; "macromolecules"
EX.
-fats, oils, phospholipids. steroids (cholesterol), waxes and terpens
Saturated Fatty Acids- comes from animal fats like lard and butter.
Unsaturated Fatty Acids- comes from the fats of plants and fishes.
Proteins
- are macromolecules that contain carbon, hydrogen, oxygen and nitrogen.
Amino acids- are necessary for protein synthesis.
Nonessential amino acids- can be synthesized by the body
Essential amino acids- cannot be synthesized by the body
Nucleis Acids
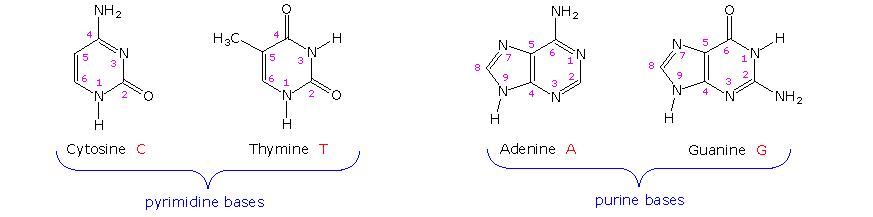
The first isolation of what we now refer to as DNA was accomplished by Johann Friedrich Miescher circa 1870. He reported finding a weakly acidic substance of unknown function in the nuclei of human white blood cells, and named this material "nuclein". A few years later, Miescher separated nuclein into protein and nucleic acid components. In the 1920's nucleic acids were found to be major components of chromosomes, small gene-carrying bodies in the nuclei of complex cells. Elemental analysis of nucleic acids showed the presence of phosphorus, in addition to the usual C, H, N & O. Unlike proteins, nucleic acids contained no sulfur. Complete hydrolysis of chromosomal nucleic acids gave inorganic phosphate, 2-deoxyribose (a previously unknown sugar) and four different heterocyclic bases (shown in the following diagram). To reflect the unusual sugar component, chromosomal nucleic acids are called deoxyribonucleic acids, abbreviated DNA. Analogous nucleic acids in which the sugar component is ribose are termed ribonucleic acids, abbreviated RNA. The acidic character of the nucleic acids was attributed to the phosphoric acid moiety.
The two monocyclic bases shown here are classified as pyrimidines, and the two bicyclic bases are purines. Each has at least one N-H site at which an organic substituent may be attached. They are all polyfunctional bases, and may exist in tautomeric forms.
Base-catalyzed hydrolysis of DNA gave four nucleoside products, which proved to be N-glycosides of 2'-deoxyribose combined with the heterocyclic amines. Structures and names for these nucleosides will be displayed above by clicking on the heterocyclic base diagram. The base components are colored green, and the sugar is black. As noted in the 2'-deoxycytidine structure on the left, the numbering of the sugar carbons makes use of primed numbers to distinguish them from the heterocyclic base sites. The corresponding N-glycosides of the common sugar ribose are the building blocks of RNA, and are named adenosine, cytidine, guanosine and uridine (a thymidine analog missing the methyl group).
From this evidence, nucleic acids may be formulated as alternating copolymers of phosphoric acid (P) and nucleosides (N), as shown:
~ P – N – P – N'– P – N''– P – N'''– P – N ~
At first the four nucleosides, distinguished by prime marks in this crude formula, were assumed to be present in equal amounts, resulting in a uniform structure, such as that of starch. However, a compound of this kind, presumably common to all organisms, was considered too simple to hold the hereditary information known to reside in the chromosomes. This view was challenged in 1944, when Oswald Avery and colleagues demonstrated that bacterial DNA was likely the genetic agent that carried information from one organism to another in a process called "transformation". He concluded that "nucleic acids must be regarded as possessing biological specificity, the chemical basis of which is as yet undetermined." Despite this finding, many scientists continued to believe that chromosomal proteins, which differ across species, between individuals, and even within a given organism, were the locus of an organism's genetic information.
It should be noted that single celled organisms like bacteria do not have a well-defined nucleus. Instead, their single chromosome is associated with specific proteins in a region called a "nucleoid". Nevertheless, the DNA from bacteria has the same composition and general structure as that from multicellular organisms, including human beings.
Views about the role of DNA in inheritance changed in the late 1940's and early 1950's. By conducting a careful analysis of DNA from many sources, Erwin Chargaff found its composition to be species specific. In addition, he found that the amount of adenine (A) always equaled the amount of thymine (T), and the amount of guanine (G) always equaled the amount of cytosine (C), regardless of the DNA source. As set forth in the following table, the ratio of (A+T) to (C+G) varied from 2.70 to 0.35. The last two organisms are bacteria.
Bwahahahaha! Does this mean that the one that doesn't belong to the following group jiggy in our Advance Biology test earlier is (A+U) ? Haha! I see. :)
To make this short, 5-carbon sugar linked together makes a nucleoside, then, it turns into nucleotide. Since the nucleotide is the building block of the Nucleic Acid thus Nucleic Acid is formed.
;)
VI. Inorganic Compounds
* Water (H20)
* Biological Solvent
* High heat capacity
* High heat fusion
* High heat vaporization
* Lubricant
* Moisturizers
* means for transport
* medium for chemical
* chemical processes
Water is formed by the process of Hydrogen Bond.
Acids + Bases= Biological Buffers
Buffers- a mixture of weak and its corresponding base that controls the Ph of a given substance.
Ph- Potential Hydrogen
| Acids are chemicals which will turn litmus paper red. Litmus is a coloured chemical which can change from red to blue and back again. Which colour it is depends upon the concentration of Hydrogen ions.If the concentration of Hydrogen ions is higher than it is in pure water then the litmus will turn red. If it is lower than in pure water the litmus will turn blue. |
A base is any compound that yields hydroxide ions (OH-) when dissolved in water. There are quite a few identifiable bases with hydroxide in the the formula such as sodium hydroxide (NaOH) and magnesium hydroxide (Mg(OH)2).
That is all.
♥Jeyonce♥

No comments:
Post a Comment